CGHS: Sealed Rate Quotation supply
Subject: Sealed Rate Quotation for Anti-Cancer Drugs and other Restricted Drugs. Regarding.
Sealed Rate Quotations are invited from Manufacturer/Importer of Anti-Cancer and other restricted Drugs for supply to CGHS for its beneficiaries on case to case basis for a period of 6 months, extendable by another 6 months or till the finalization of tender by MSO whichever is earlier. The list of the 27 (Twenty Seven) drugs/items is placed at Annexure A.
1. Eligibility Criteria: The eligibility criteria are as follow:-
2. Terms and Conditions:
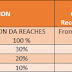
5. Critical Date Sheet
CGHS
F.No.30-02/2017-18/CGHS/MSD/LS/1347-55
Government of India
0/o the Additional Director
CGHS Medical store Depot
DIZ Area, Sec. II, Udyan Marg, Gole Market
New Delhi 110001
Government of India
0/o the Additional Director
CGHS Medical store Depot
DIZ Area, Sec. II, Udyan Marg, Gole Market
New Delhi 110001
Date: 26-07-2018
SEALED RATE QUOTATION
Sealed Rate Quotations are invited from Manufacturer/Importer of Anti-Cancer and other restricted Drugs for supply to CGHS for its beneficiaries on case to case basis for a period of 6 months, extendable by another 6 months or till the finalization of tender by MSO whichever is earlier. The list of the 27 (Twenty Seven) drugs/items is placed at Annexure A.
1. Eligibility Criteria: The eligibility criteria are as follow:-
a. The manufacturer/importer should be a holder of valid applicable licenses.
b. The manufacturer/importer should be holder of a valid WHO-GMP/GMP certific4te as per revised schedule-M of Drugs and Cosmetic Act.
c. The manufacturer/importer should not be currently blacklisted/debarred from any Govt. Organization.
d. The manufacturer/importer should have PAN Card/GST Registered.
2. Terms and Conditions:
a. The supplies shall be made on credit basis.3. EMD & PBG:
b. The payment shall be made on receipt of pre-receipted invoice in the name of “Additional Director (Medical Store Depot), CGHS. Gole Market, DIZ Area, Udyan Marg, New Delhi.
c. Manufacturer/Importer shall bear all taxes/GST or any other relevant tax as required under law, on aforesaid supplies.
d. No substitution/alternative drug will be accepted under any circumstance.
e. The supplies shall be covered under “Fall Clause” wherein the manufacturer/Importer will undertake that price quoted to MSD, Delhi is the lowest rate, offered to any institution (Private or Govt.) and in case there is fall in institutional price, they shall reduce the price accordingly, or if they sell or offer to sell such stores to any other party at a rate lower than the price charged they will forthwith notify such reduction.
f. Any reduction of Tax rates shall also be passed on to CGHS.
g. The shelf life of drugs supplied should not have passed more than 1/6 of the total shelf life at the time of supply of drugs. In case of imported drugs, the shelf life should not have crossed more than 1/4 of total shelf life. However on case to case basis the lower shelf life may be accepted, subject to the furnishing of an undertaking that if any quantity remains unconsumed, the same shall be replaced or cost of drug shall be refunded.
h. Supplies of drugs are to be made on next working day at MSD, CGHS, Gole Market, New Delhi & on 3rd working day at NCR CGHS Wellness centres of Ghaziabad, Faridabad, and Gurgaon & Noida or at places as decided by competent authority.
i. The drugs should be supplied with strict cold chain, if required.
j. In case the manufacturing of a drug has been stopped by the manufacturer, it will be informed well in advance, preferably three months prior.
k. The manufacturer/importer will not supply any drug, not approved by the Drug Controller of India or which has been subsequently derecognized by the Drug Controller. In case, such an instance is found out, no payment will be made for such supply and penalty will be imposed as per approval of competent authority along with legal proceeding as per rule, if applicable.
l. No Commitment to accept best or any other offer: CGHS shall be under no obligation to accept the best or any other offer received in response to this sealed rate quotation notice and shall be entitled to reject any or all the quotations including those received late or incomplete quotations without assigning any reason whatsoever. CGHS will not be obliged to meet and have discussions with any company/importer, and/or to listen to any representation. While the above procedures lay down the overall guidelines, CGHS reserves the right to select the company/importer based on other parameters at its discretion.
m. Conditional offers and non-conformity of the terms and conditions and offers not submitted as per the details, will be rejected summarily.
n. Splitting clause: CGHS reserves the right to split orders in case of same rates for same drug offered by different Manufacturer/importer.
o. Successful manufacturer/Importer whose rates are accepted will have to Supply the medicines as per “Annexure A”.
p. CGHS reserves the right to omit/remove any drug from drug list in the event the drug is available indigenously or if directed by competent authority.
q. In case of termination, CGHS has the right to extend the offer to L2 Manufacturer/Importer at Ll price.
a. EMD of an amount of Rs. 50,000/-(Fifty Thousand rupees) in the form of Bank Guarantee has to be submitted along with the quotation and same shall be returned to unsuccessful manufacturer/importer.4. Submission of documents: the Manufacturer/Importer shall submit the following documents:
b. The Manufacturer/importer declared successful has to submit a PBG of an amount of Rs. 5,00,000/-(Five Lakh rupees) valid for 6 months beyond expiry of Contract. EMD will be returned on receipt of Performance bank Guarantee.
a. Unconditional Acceptance Letter of Terms and conditions of rate enquiry.
b. EMD for an amount of Rs. 50,000/- (Fifty Thousand Rupees) in the form of Bank guarantee.
c. Fall Clause: That “We the manufacturer/Importer hereby undertake that price quoted to MSD, Delhi is the lowest rate, offered to any institution (Private or Govt). In case there is fall in institutional price, we will reduce the price accordingly, or if we sell or offer to sell such stores to any other party at a rate lower than the price charged we will forthwith notify such reduction.”
d.A self-attested copy of valid manufacturing license/import license.
e. A self-attested copy of WHO-GMP/GMP certificate as per revised scheduled- M of Drug and Cosmetic Act.
f. If supplies are to be made through an authorized distributor, then authorization letter from manufacturer/importer of drug along with relevant retail/wholesale drug license of the stockist/distributor.
g. A self - attested copy of PAN/GST registration of manufacturer/importer/authorized distributor.
h. Proprietary Article certificate, if applicable.
i. Mandate Form (Annexure B), Vendor Detail form (Annexure C).
j. Duly filled quotation in the Format provided below on the letterhead to be signed and stamped by the authorized signatory.
| S.No | Generic Name | Brand Name | Strength | Unit/Pack | MRP (Rs.) | Rate offered to CGHS (Exclusive of GST) | GST Rate | Net Rate offered to CGHS (inclusive of GST) |
|---|---|---|---|---|---|---|---|---|
a. Date of Issue of Notice Inviting Quotation: 26/07/20186. Terms & conditions of supplies:
b. Last date of submission: 09/08/2018, 12:00 PM
c. Date of Opening of Sealed Rate Quotations: 3:00 PM on 09/08/2018.
a. Online supply order shall be placed upon the supplier, declared successful, by CGHS upon receipt of Indent from various CGHS Wellness Centres and online access shall be provided to the supplier in this regard.7. Penalties and other important Terms and conditional:
b. Supply confirmation shall be provided by the supplier upon delivery of goods to CGHS MSD.
a. CGHS has the right to recover penalties or any other loss occurred from the submitted PBG/pending bills of Manufacturer/Importer.This is issued with the approval of the competent authority.
b. Fall clause: In case a firm is found to be in violation of the aforementioned fall clause, recovery shall be made from the existing bills of Manufacturer/importer/Authorized Agent of manufacturer and any decision in this regard by CGHS will be final.
c. Termination clause: CGHS reserves the right to terminate the Rate Quotation, if the execution of work is unsatisfactory or the time schedule is not strictly adhered to.
d. In case of termination, CGHS has the right to extend the offer to L2 Manufacturer/Importer at L1 price.
e. Liquidated Damages: If the Manufacturer/Importer/Authorized Agent of manufacturer fails to deliver the goods within the prescribed Delivery Period, the CGHS has the right to recover liquidated damage equivalent to 0.5% per day thereof of the value of the delayed stores subject to a ceiling of 5% of value of delayed stores.
f. CGHS also reserves the right to report the Manufacturer/Importer to The State/National Drug Authorities recommending punitive action against the firm for violations of terms & conditions.
g. CGHS may, without prejudice to any other remedy for breach of Terms and Conditions of rate Enquiry, by written notice of default sent to the Manufacturer/Importer/Authorized Agent of manufacturer, terminate the Rate Enquiry in whole or part
i. If the successful Manufacturer/Importer/Authorized Agent of manufacturer fails to provide any or all ‘of the services within the period(s) specified in the Sealed Rate Enquiry
ii. If the successful Manufacturer/Importer/Authorized Agent of manufacturer fails to perform any other obligation(s) under the Terms and Conditions of Sealed rate Enquiry including not abiding by all statutory liabilities under Statutory Laws.
iii. If the Manufacturer/Importer/Authorized Agent of manufacturer, in the judgment of the CGHS has engaged in corrupt or fraudulent practices in competing for or in executing the Supply of Drugs including sub-contracting or in contravention of Code of Integrity.
h. AD (MSD), CGHS, Delhi reserves the right to cancel any or all quotations without assigning any reason.
CMO(Drugs)
CGHS,Medical Store Depot
New Delhi
CGHS,Medical Store Depot
New Delhi
Annexure - A
| S.No | Medicine Name | Type | Strength |
|---|---|---|---|
| 1 | CLOSTRIDIUM BOTULINEM TYPE A (500 IU) | INJ | 500 IU |
| 2 | DACLATASVIR (60 MG) | TAB | 60 MG |
| 3 | DECITABINE (50 MG) | INJ | 50 MG |
| 4 | ENZALUTAMIDE | CAP | 40 MG |
| 5 | INTERFERON BETA IA (44 MCG) | INJ | 44 MCG |
| 6 | IVIG (5 GM) | INJ | 5 GM |
| 7 | LEDIPASVIR AND SOFOSBUVIR | TAB | 90 MG + 400 MG |
| 8 | METHOXY POLYETHYLENE GLYCOL - EPOETIN BETA (100 MCG) | INJ | 100 MCG |
| 9 | METHOXY POLYETHYLENE GLYCOL - EPOETIN BETA (50 MCG) | INJ | 50 MCG |
| 10 | METHOXY POLYETHYLENE GLYCOL - EPOETIN BETA (75 MCG) | INJ | 75 MCG |
| 11 | OCTREOTIDE (30 MG) | INJ | 30 MG |
| 12 | POSACONAZOLE (40 MG) | SYP | 40 MG |
| 13 | PEG INTERFEROW ALPHA 2B (80 MCG) | INJ | 80 MCG |
| 14 | POMALIDOMIDE (1 MG) | CAP | 1 MG |
| 15 | POMALIDOMIDE (2 MG) | CAP | 2 MG |
| 16 | POMALIDOMIDE (4 MG) | CAP | 4 MG |
| 17 | REGORAFENIB (40 MG) | TAB | 40 MG |
| 18 | RECOM INTER BETA 1A (30 MCG) | INJ | 30 MCG |
| 19 | RANIBIZUMAB | INJ | 2.3 MG |
| 20 | SOMATROPIN (36 IU) | INJ | 36 IU |
| 21 | SOMATROPIN ( 15 IU) | INJ | 15 IU |
| 22 | SOMATROPIN (45 IU) | INJ | 45 IU |
| 23 | SOFOSBUVIR (400 MG) | TAB | 400 MG |
| 24 | SOFOSBUVIR 400MG + VELPATASVIR 100MG) | TAB | 400 MG + 100 MG |
| 25 | TRASTUZUMAB (150 MG) | INJ | 150 MG |
| 26 | TRASTUZUMAB (440 MG) | INJ | 440 MG |
| 27 | TRETINOIN (10 MG) | CAP | 10 MG |
- MANDATE FORM FOR COMPANIES
- Vendor Details Form





0 comments:
Post a Comment